THEORETICAL STUDIES OF CLUSTERING, IONIZATION, AND COMPLEXATION PROCESSES IN LIQUID, VAPOR, AND WATER NANODROPLETS
Seminars
Semester 2
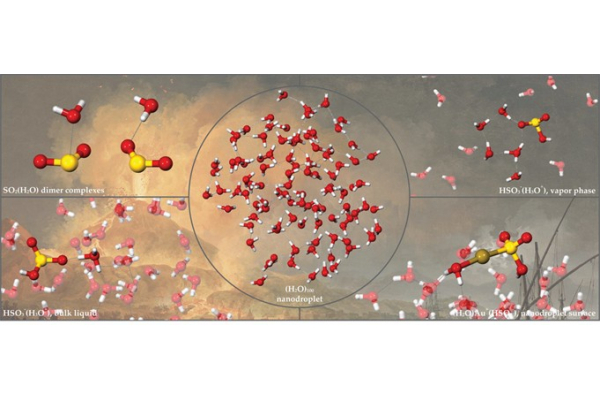
Water hydrogen bonding underlies the ice, liquid, and steam phases, while also gives rise to nano- scale structures such as nanodroplets. In dense hydrothermal vapors, water nanodroplets form through nucleation, exhibiting distinct surface and interior densities that influence the dissolution and mobility of volatiles and metals. To better understand the role of aqueous nanodroplets as a hydrothermal transport media, this theoretical study explores fluid properties of (H2O)n nanodroplets, and their interactions with sulfur and gold species, key findings include: i) CCSD(T) calculations reveal that the stacked SO2(H2O) dimer exhibits a 100% higher binding energy D0 than the hydrogen-bonded configuration, suggesting greater stability for the stacked dimer motif; ii) CPMD thermodynamic integration reveals increasing ionization energy (∆G) and acidity constants (pKa) for H2SO3 at 25°C to 350°C, with vapor-phase H2SO3 ionization at 350°C requiring ~30% higher in energy compared to bulk liquid, AIMD simulation of H2SO3 show greater resistance against ionization at the nanodroplet surface than in the interior region; iii) Classical simulations reveal that (H2O)100 nanodroplet interiors reach pressures of 2200 bar at 25°C, while surface regions transition to vapor-like densities. AIMD simulations predict comparable gold(I)-bisulfite complexation strengths in bulk liquid (∆G=17.6 kcal/mol) and droplet interiors (18.3 kcal/mol), yet enhanced AuHSO3 complexation at droplet surface (20.2 kcal/mol); iv) AIMD simulations indicate minimal evaporation from (H2O)100 nanodroplet up to 250°C (≤13 H2O), but rapid loss (50 H2O) at 300°C, with denser vapors hindering evaporation. Surface water ionization remains less favorable, with ∆G increasing from 27.8 kcal/mol at 25°C to 32.3 kcal/mol at 250°C. These findings highlight nanodroplets as distinct fluid environments from conventional bulk liquid and vapor phases, and may play a critical role in driving dissolution and the mobility of volatile and metal species in volcanic and hydrothermal systems.
Additional information: Mr. Wallace HUI, wallh817@connect.hku.hk